CMS published an OPPS final rule in the Federal Register which contains important information for several different programs, including OAS CAHPS and the Radiation Oncology (RO) Model.
OAS CAHPS
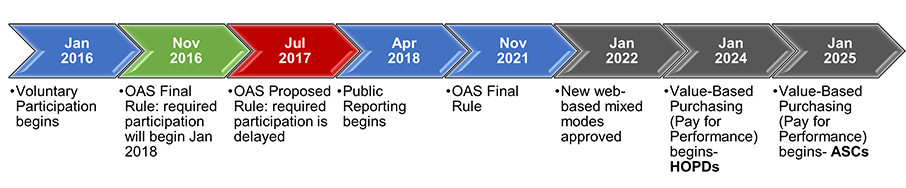
After a successful mode experiment in 2019, CMS finalized their approval of two new mixed-modes of survey administration that include a web component (web-mail and web-telephone) as of January 1, 2022. Both modes would use email invitations to contact patients initially and then follow up with non-respondents by mail or phone, respectively. They intend that these methodologies would place a lower financial burden on hospitals and ambulatory surgery centers.
CMS also finalized their timeline of mandatory OAS surveying. The CY2023 OAS CAHPS data submission will remain voluntary, and mandatory reporting will begin with CY2024 for hospital-based outpatient departments (HOPDs) (affecting reimbursement for the CY2026 payment determination). However, mandatory reporting of OAS data will begin for ambulatory surgery centers (ASCs) a year later, in CY2025.
HOPDs will be required to receive at least 300 completed surveys per year, however, ASCs will have a reduced target number of completes of only 200 surveys per year.
This final rule discusses the continued use of CPT codes to determine eligibility, and encourages facilities with delays to CPT coding to determine alternative ways to identify patients who received eligible procedures in HOPDs or ASCs.
Radiation Oncology (RO) Model
After delays due to the pandemic, CMS has finalized the proposal to begin the 5-year RO model on January 1, 2022, which would include the CAHPS® Cancer Care Radiation Therapy Survey as part of an aggregate quality score that would drive pay-for-performance for this service. CMS is also adding an Extreme and Uncontrollable Circumstances (EUC) policy to allow CMS to respond to circumstances (including the COVID-19 Public Health Emergency) that interfere with participating facilities being able to follow the requirements of the RO model.
Radiation oncology providers should not experience any additional cost as a result of implementation of the survey—CMS will pay to pull the sample and conduct the surveys through a selected contractor, so at this time, PRC (as well as other survey vendors) will not be approved survey vendors as CMS is conducting the surveys.
This model is designed for physician group practices (PGPs), freestanding radiation therapy centers, or Hospital Outpatient Departments (HOPDs), and participation is required for providers within randomly selected areas. CMS has provided a list of the zip codes required to participate here. The RO model will include approximately 30% of all eligible RO episodes nationally. The newly published final rule excludes particular types of facilities from the model, such as:
- HOPDs participating in (Pennsylvania Rural Health Model) PARHM. HOPDs eligible to participate in PARHM that are not actively participating in PARHM are still included in the RO Model.
- HOPDs of any hospital in the Community Transformation Track of the Community Health Access and Rural Transformation (CHART) Model.
The types of cancer included in the model are commonly treated with radiation therapy and have pricing stability. The rule finalizes removing liver cancer from the RO Model because evidence-based clinical treatment guidelines do not support its treatment with radiation.
The rule also finalizes removing brachytherapy as a modality of delivering radiation from the model, but CMS intends to continue to monitor its use and potentially include it in the RO model in the future.
For more information, please reference the CMS website for this model, and contact us with any further questions.


